-
Tarsus Pharmaceuticals, Inc. Announces Positive Results of Saturn-1 Pivotal Trial Evaluating TP-03 for the Treatment of Demodex Blepharitis
ソース: Nasdaq GlobeNewswire / 21 6 2021 05:00:00 America/Chicago
Saturn-1 Phase 2b/3 trial met all primary and secondary endpoints, and demonstrated significant, clinically meaningful outcomes with no serious treatment-related adverse events and no treatment-related discontinuations, demonstrating the potential of TP-03 to treat Demodex blepharitis, a disease with no FDA-approved therapies
As many as 25 million Americans may have Demodex blepharitis, an ocular disease that can have a significant clinical burden and negatively impact patients’ daily lives
Conference call and webcast scheduled for today at 8:00 a.m. ET to review detailed topline Saturn-1 data
IRVINE, Calif., June 21, 2021 (GLOBE NEWSWIRE) -- Tarsus Pharmaceuticals, Inc. (NASDAQ: TARS), a late clinical-stage biopharmaceutical company whose mission is to focus on unmet needs and apply proven science and new technology to revolutionize treatment for patients, starting with eye care, today announced that all pre-specified primary and secondary endpoints were met for its pivotal Phase 2b/3 Saturn-1 trial evaluating the company’s novel investigational therapeutic, TP-03 (lotilaner ophthalmic solution, 0.25%), in patients with Demodex blepharitis. Results demonstrated a statistically significant complete collarette cure at day 43 in patients with Demodex blepharitis treated with TP-03 compared to vehicle (p<0.0001; primary endpoint). The Saturn-1 trial also met the secondary endpoints of mite eradication at day 43 (p<0.0001) and composite cure based on complete collarette and erythema cures at day 43 (p<0.0001). In addition, significant, clinically meaningful improvements were observed within two weeks across multiple endpoints. TP-03 was well tolerated with a safety profile similar to vehicle, and there were no treatment-related discontinuations.
“Millions of people are living with Demodex blepharitis, and we know from recent research that these patients are suffering daily. With no U.S. Food and Drug Administration (FDA)-approved therapies, both patients and eye care professionals need a solution to eradicate the mites that cause the disease,” said Bobak Azamian, M.D., Ph.D., President and Chief Executive Officer of Tarsus. “We believe the results from our Saturn-1 trial mark an important moment in Demodex blepharitis research, showing the potential of TP-03 to target the underlying cause of this disease and potentially become the standard of care for patients and clinicians. We expect to provide topline results for our second pivotal trial for TP-03, Saturn-2, in Q1 of 2022. If Saturn-2 trial data is positive, similar to the positive Saturn-1 results, we expect both Saturn-1 and Saturn-2 trials to support our submission of a New Drug Application (NDA) for TP-03 for the treatment of Demodex blepharitis in 2022.”
Demodex blepharitis is a highly prevalent ocular disease, affecting as many as 25 million Americans, that can have a significant clinical burden and negatively impact patients’ daily lives. The disease is caused by an infestation of Demodex mites, the most common ectoparasite found on humans, that live on the skin of the face and eyelids. Demodex blepharitis is characterized by inflammation of the eyelid margin, redness and ocular irritation. TP-03 has the potential to be the first FDA-approved therapeutic for Demodex blepharitis and targets the underlying cause of disease – Demodex mite infestation. The Saturn-1 trial is the first large-scale trial to show positive, clinically meaningful results for a therapeutic specifically designed to treat Demodex blepharitis.
Saturn-1 Phase 2b/3 Results
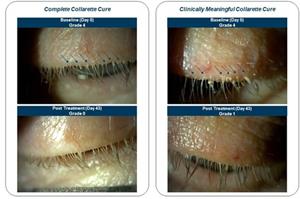
Results demonstrated 81% of patients achieved a significant, clinically meaningful collarette cure defined by a collarette grade of zero (0) or one (1) at day 43 compared to 23% of those on vehicle (p<0.0001). Additionally, a significant, clinically meaningful collarette cure was seen in 23% of patients on TP-03 compared to 11% on vehicle as early as day 8 (p=0.0003). Saturn-1 data also showed that 43% of patients on TP-03 achieved the primary endpoint of complete collarette cure (grade 0) at day 43, defined as zero to two (0-2) collarettes per lid compared to 7% on vehicle (p<0.0001). Collarettes, a pathognomonic sign of Demodex infestation, are composed of partially digested epithelial cells, mite waste products and eggs and are most easily observed at the base of the upper eyelashes when the patient looks down during a standard eye examination.
The secondary endpoint of complete mite eradication achieved statistically significant results by day 15, and 68% of patients on TP-03 achieved mite eradication compared to 18% on vehicle (p<0.0001) at day 43. Mite eradication is defined as a mite density of zero (0) mites per lash.
For composite cure, 68% of patients experienced a significant, clinically meaningful cure of both a grade zero (0) or one (1) collarette and erythema score at day 43 compared to 20% on vehicle (p<0.0001), with significant improvements seen as early as day 8. Additionally, 13.4% of patients on TP-03 achieved a complete composite cure, which was another secondary endpoint, based on a composite of collarette cure and erythema cure compared to 1.0% on vehicle (p<0.0001) at day 43. Composite cure is defined as the presence of zero to two (0-2) collarettes on the upper eyelid and the absence of erythema (redness). Results for complete erythema cure (19% of patients on TP-03 compared to 7% of patients on vehicle, p<0.0001) and one (1) grade or more erythema improvement (45% of patients on TP-03 compared to 28% of patients on vehicle, p=0.0002) were also statistically significant.
Trial Safety Data
TP-03 is a well-characterized anti-parasitic agent that paralyzes and eradicates Demodex mites by selectively inhibiting parasite-specific GABA-Cl channels. Saturn-1 trial results demonstrated that TP-03 was well tolerated with a safety profile similar to the vehicle group. Additionally, most TP-03 patients (92%) reported that the drop comfort was neutral to very comfortable. There were no serious treatment-related adverse events nor any treatment-related adverse events leading to treatment discontinuation. All treatment-related ocular adverse events in the TP-03 group were mild with the most common being instillation site pain/burning/stinging (11.8%, n=25). Other adverse events occurring at a rate of ≥1% in the TP-03 group included instillation site pruritis, reduced visual acuity, eye pain and eye discharge, each representing 1.4% (n=3) of patients.
Saturn-1 Phase 2b/3 Trial Design
Saturn-1 was a randomized, controlled, multicenter, double-masked trial evaluating the safety and efficacy of TP-03 in adults with Demodex blepharitis. The trial enrolled 421 adults aged 18 and over having more than 10 collarettes on the upper lid and at least mild erythema of the upper eyelid margin. Each patient had at least 1.5 mites per lash on the upper and lower eyelids combined. One drop of TP-03 was self-administered twice per day in each eye for six weeks and patients were instructed not to touch or rub their lid margin. Enrolled patients received no treatment for blepharitis symptoms (i.e., lid hygiene) during the trial or 14 days prior to enrollment.
“Demodex blepharitis is a widespread, yet frequently overlooked condition that can negatively impact the quality of life for many patients and lead to more serious health outcomes if left untreated,” said Elizabeth Yeu, M.D., Chief Medical Advisor for Tarsus. “I am highly encouraged by the results seen in the Saturn-1 trial and I’m hopeful that there may be a treatment option on the horizon that targets the underlying cause of this disease to help patients finally find relief.”
Tarsus is also evaluating TP-03 in its pivotal Saturn-2 (Phase 3) trial, which has the same endpoints as Saturn-1, and commenced patient enrollment in May of 2021. Tarsus expects topline results for the Saturn-2 trial in Q1 2022, and, if the results are similarly positive, Tarsus expects data from both the Saturn-1 and Saturn-2 trials to support submission of a New Drug Application (NDA) to the FDA for TP-03 for the treatment of Demodex blepharitis. TP-03 has the potential to help millions of patients and eye care professionals struggling to manage Demodex blepharitis.
Conference Call and Webcast Information
A detailed summary of the Saturn-1 findings will be presented on a conference call and live, listen-only webcast today at 8:00 a.m. ET. The dial-in numbers are (833) 540-1160 for domestic callers and (929) 517-0351 for international callers. The Conference ID is 3766845. The webcast of the conference call can be accessed at https://edge.media-server.com/mmc/p/uh6zebmu. After the live webcast, the event will remain archived on the Tarsus Pharmaceuticals website at https://ir.tarsusrx.com/ for 90 days.
About TP-03
TP-03 (lotilaner ophthalmic solution, 0.25%) is a novel, investigational therapeutic designed to target and eradicate Demodex mites. TP-03 is a topical ophthalmic formulation of lotilaner, which is a well-characterized anti-parasitic agent that paralyzes and eradicates Demodex mites by selectively inhibiting parasite-specific GABA-Cl channels. It is a potent, non-competitive antagonist of insect and arachnid GABA-Cl channels and a highly lipophilic molecule, which may promote its uptake in the oily sebum of the hair follicle where the mites reside. Tarsus has completed four Phase 2 clinical trials of TP-03 in Demodex blepharitis, all of which met their respective endpoints with no significant adverse events nor any events leading to treatment discontinuation. TP-03 was also evaluated in the pivotal Saturn-1 (Phase 2b/3) trial and met all primary and secondary endpoints with no serious treatment-related adverse events and no treatment-related discontinuations. It is currently being evaluated in the Saturn-2 (Phase 3) pivotal trial. If approved, TP-03 may offer treatment for millions of patients around the world with Demodex blepharitis.About Demodex Blepharitis
Blepharitis is a common ocular condition that is characterized by inflammation of the eyelid margin, redness and ocular irritation. Demodex blepharitis is caused by infestation of Demodex mites, the most common ectoparasite found on humans. Demodex mites cause approximately 45% of blepharitis, or about 9 million cases in the U.S. and the number may be as high as approximately 25 million based on Tarsus’ internal research indicating about 58% of patients presenting to eye care clinics have collarettes, a pathognomonic sign of Demodex infestation, and a published study estimating that at least 45 million people annually visit an eye care clinic. Currently, there are no FDA-approved treatments for Demodex blepharitis.About Tarsus Pharmaceuticals, Inc.
Tarsus Pharmaceuticals, Inc. is a late clinical-stage biopharmaceutical company that applies proven science and new technology to revolutionize treatment for patients, starting with eye care. It is advancing its pipeline to address several diseases with high unmet need across a range of therapeutic categories, including eye care, dermatology, and infectious disease prevention. The company is studying two investigational medicines in clinical trials. Its lead product candidate, TP-03, is a novel therapeutic being studied in a second Phase 3 pivotal trial for the treatment of Demodex blepharitis. TP-03 is also being developed for the treatment of Meibomian Gland Disease. Tarsus is developing TP-05, an oral, non-vaccine therapeutic for the prevention of Lyme disease, which is currently being studied in a Phase 1 clinical trial.Forward-Looking Statements
Statements in this press release about future expectations, plans and prospects, as well as any other statements regarding matters that are not historical facts, may constitute “forward-looking statements.” These statements includes statements regarding Tarsus’ plans for and the anticipating benefits of its product candidates, including TP-03, the timing, objectives and results of the clinical trials and anticipated regulatory and development milestones, including the timing of the Saturn-2 clinical trial and submission of an NDA, and the quotations of Tarsus’ management. The words, without limitation, “believe,” “contemplate,” “continue,” “could,” “estimate,” “expect,” “intend,” “may,” “might,” “plan,” “potential,” “predict,” “project,” “should,” “target,” “will,” or “would,” or the negative of these terms or other similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these or similar identifying words. Actual results may differ materially from those indicated by such forward-looking statements as a result of various important factors. Important factors that could cause actual results to differ materially from those in the forward-looking statements include: Tarsus has incurred significant losses and negative cash flows from operations since inception and anticipates that it will continue to incur significant expenses and losses for the foreseeable future; Tarsus may need to obtain additional funding to complete the development and any commercialization of its product candidates, if approved; Tarsus is heavily dependent on the success of its lead product candidate, TP-03 for the treatment of Demodex blepharitis; the COVID-19 pandemic may affect Tarsus’ ability to initiate and complete preclinical studies and clinical trials, disrupt regulatory activities, disrupt manufacturing and supply chain or have other adverse effects on Tarsus’ business and operations; even if TP-03, TP-05, or any other product candidate that Tarsus develops receives marketing approval, Tarsus may not be successful in educating eye care physicians and the market about the need for treatments specifically for Demodex blepharitis, Lyme disease, and/or other diseases or conditions targeted by Tarsus’ products; the development and commercialization of Tarsus products is dependent on intellectual property it licenses from Elanco Tiergesundheit AG; Tarsus will need to develop and expand the company and Tarsus may encounter difficulties in managing its growth, which could disrupt its operations; the sizes of the market opportunity for Tarsus’ product candidates, particularly TP-03 for the treatment of Demodex blepharitis and MGD, as well as TP-05 for the treatment of Lyme disease, have not been established with precision and may be smaller than estimated; the results of Tarsus’ earlier studies and trials may not be predictive of future results; any termination or suspension of, or delays in the commencement or completion of, Tarsus’ planned clinical trials could result in increased costs, delay or limit its ability to generate revenue and adversely affect its commercial prospects; and if Tarsus is unable to obtain and maintain sufficient intellectual property protection for its product candidates, or if the scope of the intellectual property protection is not sufficiently broad, Tarsus’ competitors could develop and commercialize products similar or identical to Tarsus’ products. Further, there are other risks and uncertainties that could cause actual results to differ from those set forth in the forward-looking statement and they are detailed from time to time in the reports Tarsus files with the Securities and Exchange Commission, including Tarsus’ Form 10-K for the year ended December 31, 2020 filed with the SEC on March 31, 2021 and Form 10-Q for the quarter ended March 31, 2021 filed with the SEC on May 11, 2021, which Tarsus incorporates by reference into this press release and copies of which are posted on its website and are available from Tarsus without charge. However, new risk factors and uncertainties may emerge from time to time, and it is not possible to predict all risk factors and uncertainties. Accordingly, readers are cautioned not to place undue reliance on these forward-looking statements. Any forward-looking statements contained in this press release are based on the current expectations of Tarsus’ management team and speak only as of the date hereof, and Tarsus specifically disclaims any obligation to update any forward-looking statement, whether as a result of new information, future events or otherwise.Contacts:
Media Contact:
SuJin Oh
Shop PR
(917) 841-5213
sujin@shop-pr.comInvestor Contact:
Patti Bank
Westwicke Partners, an ICR company
(415) 513-1284
IR@tarsusrx.comA photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/d788bb0e-ef21-4338-aa1d-dc7612611b81
Saturn-1 patients' collarettes at baseline and post treatment
Saturn-1 patients' collarettes at baseline (Day 0, Grade 4) and post treatment (Day 43, Grade 0, left) and (Day 43, Grade 1, right). Images demonstrate results which we believe are representative of favorable treatment with TP-03 for patients participating in the Saturn-1 trial. Other patients may experience different or less favorable results.